

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
The gas with an initial volume of 24.0 l at a pressure of 565 mmhg is compressed until the volume is...
Questions


Advanced Placement (AP), 10.09.2021 06:00


History, 10.09.2021 06:00

Mathematics, 10.09.2021 06:00

English, 10.09.2021 06:00

Mathematics, 10.09.2021 06:00

Mathematics, 10.09.2021 06:00





Mathematics, 10.09.2021 06:00



Mathematics, 10.09.2021 06:00

Chemistry, 10.09.2021 06:00

Mathematics, 10.09.2021 06:00


Mathematics, 10.09.2021 06:00

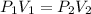
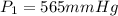
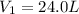
![V_2=16.0L[tex]P_2=?](/tpl/images/0084/3087/6fbf6.png)
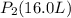
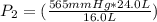
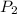 = 848 mmHg
= 848 mmHg

