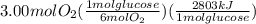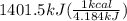Chemistry, 13.07.2019 08:00 zekrader18
The combustion of glucose (c6h12o6) with oxygen gas produces carbon dioxide and water. this process releases 2803 kj per mole of glucose. when 3.00 mol of oxygen react in this way with glucose, what is the energy release in kcal? (hint: write a balanced equation for the combustion process.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 23.06.2019 04:20
Malia was able to make a paper clip float on the surface of water what will most likely happen to the paper clip if a drop of dishwashing detergent is added near it
Answers: 1

Chemistry, 23.06.2019 06:40
4786 joules of heat are transferred to a 89.0 gramsample of an unknown material, with an initialtemperature of 23.0°c. what is the specific heat of thematerialif the final temperature is 89.5 °c?
Answers: 1

Chemistry, 23.06.2019 09:00
What sources of error may have contributed to the percent yield not being 100 percent? think about things that may have led to inaccurate measurements or where mass of the product could have been lost if this experiment was conducted in a physical laboratory.
Answers: 2
You know the right answer?
The combustion of glucose (c6h12o6) with oxygen gas produces carbon dioxide and water. this process...
Questions

Mathematics, 19.07.2019 11:10





Mathematics, 19.07.2019 11:10

Biology, 19.07.2019 11:10


History, 19.07.2019 11:10



Chemistry, 19.07.2019 11:10

Mathematics, 19.07.2019 11:10





Social Studies, 19.07.2019 11:10


History, 19.07.2019 11:10