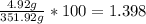Chemistry, 13.07.2019 07:30 DASASDAEDWEDA
Asolution is made containing 4.92 g of sodium lithium chloride per 347 g of water. what is the weight/weight % or percent by mass of the solute?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1


Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
Asolution is made containing 4.92 g of sodium lithium chloride per 347 g of water. what is the weig...
Questions





Mathematics, 08.11.2019 19:31

Biology, 08.11.2019 19:31


Chemistry, 08.11.2019 19:31


Mathematics, 08.11.2019 19:31

Health, 08.11.2019 19:31

Mathematics, 08.11.2019 19:31

Mathematics, 08.11.2019 19:31

Chemistry, 08.11.2019 19:31

Mathematics, 08.11.2019 19:31


Computers and Technology, 08.11.2019 19:31



Biology, 08.11.2019 19:31