
Chemistry, 21.09.2019 18:20 alondrachon
Citric acid is composed of only carbon, hydrogen, and oxygen. when a 0.5000 g sample of citric acid was burned, it produced 0.6871 g of co2 and 0.1874 g of h2o. the molar mass of the compound is 192 g/mol. what are the empirical and molecular formulas of citric acid

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2


Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
You know the right answer?
Citric acid is composed of only carbon, hydrogen, and oxygen. when a 0.5000 g sample of citric acid...
Questions







History, 26.09.2021 02:50

English, 26.09.2021 02:50


Computers and Technology, 26.09.2021 02:50

Spanish, 26.09.2021 02:50



Mathematics, 26.09.2021 02:50

Mathematics, 26.09.2021 02:50


Mathematics, 26.09.2021 03:00

English, 26.09.2021 03:00

English, 26.09.2021 03:00

Mathematics, 26.09.2021 03:00

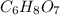 is empirical formula as well as molecular formula.
is empirical formula as well as molecular formula.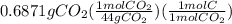
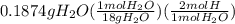
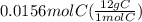
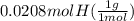
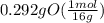
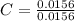 = 1
= 1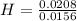 = 1.33
= 1.33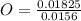 = 1.17
= 1.17

