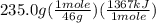Chemistry, 13.07.2019 01:00 marvinc5603
The combustion of one mole of liquid ethanol, ch3ch2oh, produces 1367 kj of heat. calculate how much heat is produced when 235.0 g of ethanol are combusted.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:30
Forests and meadows are often cut down to make way for farms or large number of new homes. what are some of the elements of ecosystems that are lost when plants in these areas are removed?
Answers: 2

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

You know the right answer?
The combustion of one mole of liquid ethanol, ch3ch2oh, produces 1367 kj of heat. calculate how much...
Questions

Mathematics, 21.09.2020 23:01


English, 21.09.2020 23:01

Computers and Technology, 21.09.2020 23:01

Mathematics, 21.09.2020 23:01

Mathematics, 21.09.2020 23:01




Geography, 21.09.2020 23:01

Mathematics, 21.09.2020 23:01


Mathematics, 21.09.2020 23:01

Mathematics, 21.09.2020 23:01

Mathematics, 21.09.2020 23:01

Health, 21.09.2020 23:01

English, 21.09.2020 23:01

Chemistry, 21.09.2020 23:01

Mathematics, 21.09.2020 23:01

Mathematics, 21.09.2020 23:01