
Let us assume that fe(oh)2(s) is completely insoluble, which signifies that the precipitation reaction with naoh(aq) (presented in the transition) would go to completion. fe2+(aq)+2naoh(aq) → fe(oh)2(s)+2na+(aq) if you had a 0.500 l solution containing 0.0230 m of fe2+(aq), and you wished to add enough 1.29 m naoh(aq) to precipitate all of the metal, what is the minimum amount of the naoh(aq) solution you would need to add? assume that the naoh(aq) solution is the only source of oh−(aq) for the precipitation.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:10
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
Let us assume that fe(oh)2(s) is completely insoluble, which signifies that the precipitation reacti...
Questions



Biology, 20.10.2020 21:01



Business, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01

English, 20.10.2020 21:01

Chemistry, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01

Biology, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01


History, 20.10.2020 21:01


Mathematics, 20.10.2020 21:01



Health, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01


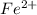 is as follows:-
is as follows:-![Fe^{2+} = 500 mL Fe^{2+} * \frac{0.0230mole\ Fe^{2+}]}{[1 mol\ Fe^{2+}}](/tpl/images/0082/8691/681c2.png) = 11.50 mmol Fe^(2+)
= 11.50 mmol Fe^(2+)![11.50\ mmol\ Fe^{2+} * \frac{[2 \ mol\ NaOH]}{[1 \mol Fe^{2+}}](/tpl/images/0082/8691/9368e.png) = 23.00 mmol NaOH
= 23.00 mmol NaOH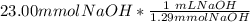 = 17.8 mL NaOH.
= 17.8 mL NaOH.


