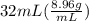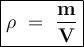Chemistry, 12.07.2019 22:30 annette211pdd8v9
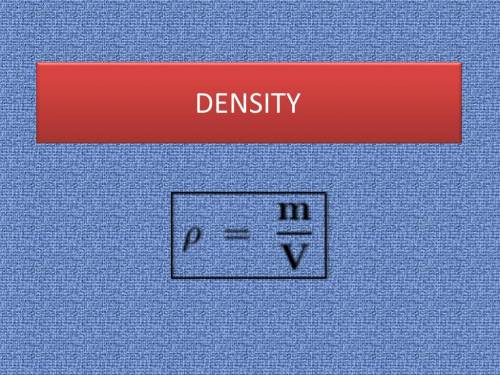
You are asked to determine the mass of a piece of copper using its reported density, 8.96 g/ml, and a 150-ml graduated cylinder. first, you add 105 ml of water to the graduated cylinder; then you place the piece of copper in the cylinder and record a volume of 137 ml. what is the mass of the copper reported with the correct number of significant figures?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2


Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
You are asked to determine the mass of a piece of copper using its reported density, 8.96 g/ml, and...
Questions

Biology, 11.11.2019 09:31


Social Studies, 11.11.2019 09:31

English, 11.11.2019 09:31

Physics, 11.11.2019 09:31





Health, 11.11.2019 09:31

Geography, 11.11.2019 09:31




English, 11.11.2019 09:31



Chemistry, 11.11.2019 09:31


Biology, 11.11.2019 09:31