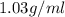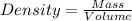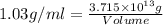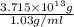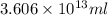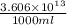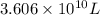Chemistry, 12.07.2019 22:00 jewelz5887
Magnesium (mg) is a valuable metal used in alloys, in batteries, and in the manufacture of chemicals. it is obtained mostly from seawater, which contains about 1.30 g of mg for every kilogram of seawater. calculate the volume of seawater (in liters) needed to extract 5.33 × 104 tons of mg. seawater has a density of 1.03 g/ml. (1 ton = 2000 lb; 1 lb = 453.6 g) enter your answer in scientific notation.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
Magnesium (mg) is a valuable metal used in alloys, in batteries, and in the manufacture of chemicals...
Questions

English, 20.07.2019 23:00

Mathematics, 20.07.2019 23:00





Social Studies, 20.07.2019 23:00









English, 20.07.2019 23:00




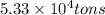 into gram.
into gram.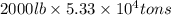
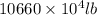 =
= 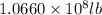
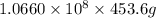
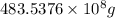 =
= 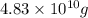
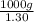 of seawater.
of seawater.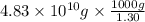 =
= 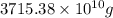
 of seawater.
of seawater.