
Chemistry, 12.07.2019 21:00 kotetravels10
Asample of an unknown substance contains 38.43 g lead, 17.83 g carbon, and 3.74 g hydrogen. what is the empirical formula? a: pbc4h10 b: pb2c8h15 c: pbc8h20 d: pb2c4h15

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
Asample of an unknown substance contains 38.43 g lead, 17.83 g carbon, and 3.74 g hydrogen. what is...
Questions

Biology, 06.10.2019 04:50

French, 06.10.2019 04:50




History, 06.10.2019 04:50

Mathematics, 06.10.2019 04:50

Physics, 06.10.2019 04:50




Biology, 06.10.2019 04:50





Mathematics, 06.10.2019 04:50

Mathematics, 06.10.2019 04:50


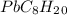 .
.

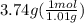
 = 1
= 1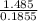 = 8
= 8 = 20
= 20

