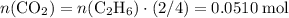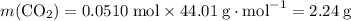Problem page gaseous ethane ch3ch3 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gaseous water h2o . suppose 0.60 g of ethane is mixed with 3.27 g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. round your answer to 2 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1


Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
Problem page gaseous ethane ch3ch3 will react with gaseous oxygen o2 to produce gaseous carbon dioxi...
Questions



Mathematics, 18.07.2019 09:30


Mathematics, 18.07.2019 09:30



Computers and Technology, 18.07.2019 09:30



Mathematics, 18.07.2019 09:30



Computers and Technology, 18.07.2019 09:30

Biology, 18.07.2019 09:30

Biology, 18.07.2019 09:30

Social Studies, 18.07.2019 09:30

Mathematics, 18.07.2019 09:30

Mathematics, 18.07.2019 09:30

History, 18.07.2019 09:30

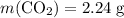
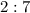 molar ratio:
molar ratio: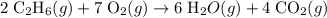
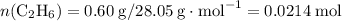
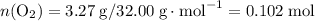
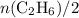 and
and 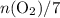 (as seen in the balanced chemical equation) shall satisfy the relationship
(as seen in the balanced chemical equation) shall satisfy the relationship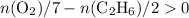
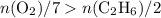
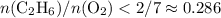
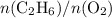 with data given in the question yields approximately
with data given in the question yields approximately 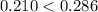 , which does satisfy the relationship. Hence the assumption holds and ethene is the limiting reactant.
, which does satisfy the relationship. Hence the assumption holds and ethene is the limiting reactant.
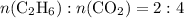 ,
,