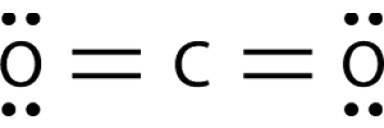Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

You know the right answer?
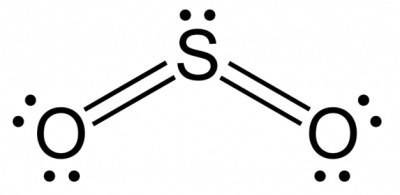
Bonds between carbon and oxygen (c=o) are more polar than bonds between sulfur and oxygen (s=o). nev...
Questions

English, 13.01.2021 07:50

Mathematics, 13.01.2021 07:50

Mathematics, 13.01.2021 07:50

Mathematics, 13.01.2021 07:50



Mathematics, 13.01.2021 07:50

Mathematics, 13.01.2021 07:50

Mathematics, 13.01.2021 07:50

Mathematics, 13.01.2021 07:50


Chemistry, 13.01.2021 07:50

English, 13.01.2021 07:50

History, 13.01.2021 07:50

Mathematics, 13.01.2021 07:50