
Chemistry, 12.07.2019 18:00 Uniquestudies
Below is a proposed two-step mechanism for the decomposition of hydrogen peroxide: h2o2(aq) + br−(aq) → h2o(ℓ) + bro−(aq) slow bro−(aq) + h2o2(aq) → h2o(ℓ) + o2(g) + br−(aq) fast is this homogeneous or heterogeneous catalysis? also identify the catalyst in the mechanism.

Answers: 1
Another question on Chemistry




Chemistry, 23.06.2019 19:30
Use the equation n2 + 3h2=2nh3 if 8.0g n2 react, how many grams of nh3 will be produced
Answers: 1
You know the right answer?
Below is a proposed two-step mechanism for the decomposition of hydrogen peroxide: h2o2(aq) + br−(a...
Questions

Chemistry, 03.12.2021 03:50


Engineering, 03.12.2021 03:50



Computers and Technology, 03.12.2021 03:50





Social Studies, 03.12.2021 03:50



Chemistry, 03.12.2021 03:50


Mathematics, 03.12.2021 03:50

Computers and Technology, 03.12.2021 03:50



History, 03.12.2021 03:50

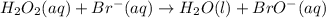 .....Slow
.....Slow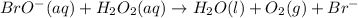 .....Fast
.....Fast

