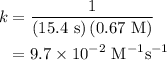Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
The half-life for the second-order decomposition of hi is 15.4 s when the initial concentration of h...
Questions








Mathematics, 05.07.2019 07:30





History, 05.07.2019 07:30


Mathematics, 05.07.2019 07:30


Mathematics, 05.07.2019 07:30



Mathematics, 05.07.2019 07:30

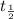 =
=![\frac{1}{k[A_o]}](/tpl/images/0081/7570/1cf43.png) (1)
(1)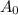 = initial concentration
= initial concentration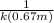
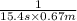
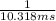
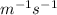
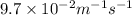
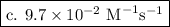 .
.
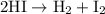
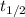 .
.
![{t_{{\text{1/2}}}} = \dfrac{1}{{k\left[ {{{\text{A}}_{\text{o}}}} \right]}}](/tpl/images/0081/7570/8ca83.png) …… (1)
…… (1)
![\left[ {{{\text{A}}_{\text{o}}}} \right]](/tpl/images/0081/7570/38db1.png) is the initial concentration of reactant.
is the initial concentration of reactant.
![k = \dfrac{1}{{{t_{{\text{1/2}}}}\left[ {{{\text{A}}_{\text{o}}}} \right]}}](/tpl/images/0081/7570/d5e88.png) …… (2)
…… (2)