

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 05:00
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2
You know the right answer?
Titanium (ti) has an hcp crystal structure, a density of 4.51 g/cm3, and the atomic weight for ti, a...
Questions



Mathematics, 02.01.2021 22:50



Mathematics, 02.01.2021 22:50



Mathematics, 02.01.2021 22:50

Mathematics, 02.01.2021 23:00





Social Studies, 02.01.2021 23:00



Advanced Placement (AP), 02.01.2021 23:00

Mathematics, 02.01.2021 23:00

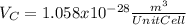
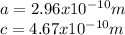
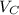 :
: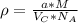
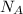 for the Avogadro number, thus:
for the Avogadro number, thus: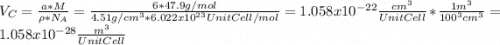
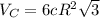
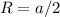 and
and 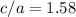 , thus:
, thus:![V_C=6c(a/2)^2\sqrt{3}\\V_C=6a*1.58(a/2)^2\sqrt{3}\\V_C=16.4a^3/4\\a=\sqrt[3]{\frac{4*1.058x10^{-28}\frac{m^3}{UnitCell}}{16.4} } \\a=2.96x10^{-10}m\\c=1.58a\\c=1.58*2.96x10^{-10}m\\c=4.67x10^{-10}m](/tpl/images/0081/7540/a224e.png)


