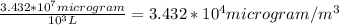Answers: 1
Another question on Chemistry



Chemistry, 23.06.2019 02:50
For questions 1 and 2, consider the following experimental data.hydrogen emission lines were detected at the following wavelengths (in nm): 121.6102.697.395.093.8question 1use the electromagnetic radiation classifications below and figure 1-1 in the introductory information for this lab (in the lab manual) to determine the nf value for the experimental data provided? wavelength, ? (nm) 650 700 550 600 400 450 500 visible spectrum wavelength, ? (m) 11 10 3 10 10 10 8 10 5 10 10 -10 10 9 10 10 10 10 -12 10 microwave radio infrared x-ray ultraviolet gamma 1020 1019 1018 1 1016 015 1014 01 12 109108 frequency, v (hz)a.1b. 2c. 3d. 4e. 5question 2using the data for the emission line with the longest wavelength, the known value of nf (from question 1 in this prelab), and the value of ni (deduced from the ? and nf values) calculate the rydberg constant for hydrogen (rh) in units of m-1.a) 1.097 x 10-11 m-1b) 5.921 x 107 m-1c) 1.097 x 10-2 m-1d) 9.252 x 106 m-1e) 1.097 x 107 m-1
Answers: 3

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
The sulfur dioxide (so2) stack-gas concentration from fossil-fuel combustion is 12 ppmv. determine t...
Questions

Arts, 26.10.2020 15:30

Mathematics, 26.10.2020 15:30


English, 26.10.2020 15:30

Mathematics, 26.10.2020 15:30

History, 26.10.2020 15:30

Arts, 26.10.2020 15:30

Mathematics, 26.10.2020 15:30

Mathematics, 26.10.2020 15:30



Mathematics, 26.10.2020 15:30




History, 26.10.2020 15:30

Mathematics, 26.10.2020 15:30

English, 26.10.2020 15:30


Mathematics, 26.10.2020 15:30

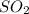 in the stack gas = 12 ppmv
in the stack gas = 12 ppmv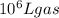
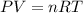
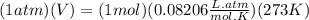
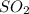 occupies 22.4 L
occupies 22.4 L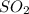 =
= 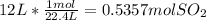
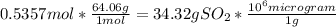 =
=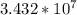 μg
μg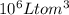 :
: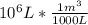 =
= 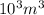
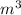 :
: