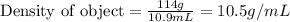In an old trunk, you find a piece of metal that you think may be aluminum, silver, or lead. you take it to a lab, where you find it has a mass of 114 g and a volume of 10.9 cm3. what is the metal you found? assume that densities of aluminium, silver, and lead are 2.70, 10.5, and 11.3 g/ml, respectively.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3
You know the right answer?
In an old trunk, you find a piece of metal that you think may be aluminum, silver, or lead. you take...
Questions


Mathematics, 27.02.2020 18:31



Chemistry, 27.02.2020 18:31








Mathematics, 27.02.2020 18:32



Mathematics, 27.02.2020 18:32





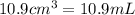 (Conversion factor:
(Conversion factor:  )
)