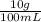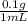Chemistry, 12.07.2019 15:00 hdwoody2002
The anesthetic procaine hydrochloride is often used to deaden pain during dental surgery. the compound is packaged as a 10.% solution (by mass; d = 1.0 g/ml) in water. if your dentist injects 0.30 ml of the solution, what mass of procaine hydrochloride (in milligrams) is injected?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
The anesthetic procaine hydrochloride is often used to deaden pain during dental surgery. the compou...
Questions

Mathematics, 24.03.2021 01:00






Mathematics, 24.03.2021 01:00



Mathematics, 24.03.2021 01:00

English, 24.03.2021 01:00


Mathematics, 24.03.2021 01:00

Biology, 24.03.2021 01:00

English, 24.03.2021 01:00

History, 24.03.2021 01:00




History, 24.03.2021 01:00