

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1
You know the right answer?
The osmotic pressure, π, of a solution of glucose is 132 atm . find the molarity of the solution at...
Questions

Mathematics, 21.05.2020 21:05

Mathematics, 21.05.2020 21:05




English, 21.05.2020 21:05





Computers and Technology, 21.05.2020 21:05








Mathematics, 21.05.2020 21:05

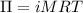
 is osmotic pressure,
is osmotic pressure,  is van't Hoff's factor,
is van't Hoff's factor, 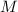 molarity,
molarity, 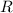 is Ideal gas constant, and T is Temperature.
is Ideal gas constant, and T is Temperature. = 132 atm
= 132 atm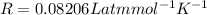
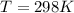
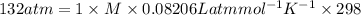
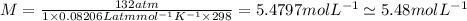
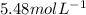 .
.

