Chemistry, 12.07.2019 12:30 markmlg122
An unknown compound with a molar mass of 155.06 g/mol consists of 46.47% c, 7.80% h, and 45.72% cl. find the molecular formula for the compound. c6h12cl2 chcl c9h18cl3 c6h12cl

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
An unknown compound with a molar mass of 155.06 g/mol consists of 46.47% c, 7.80% h, and 45.72% cl....
Questions



Mathematics, 28.09.2021 19:10


Arts, 28.09.2021 19:10

Social Studies, 28.09.2021 19:10



Computers and Technology, 28.09.2021 19:10

Mathematics, 28.09.2021 19:10


Mathematics, 28.09.2021 19:10





Social Studies, 28.09.2021 19:10

History, 28.09.2021 19:10


Mathematics, 28.09.2021 19:10

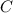 :
: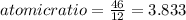
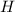 :
: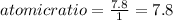
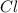 :
: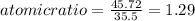
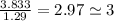
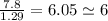
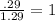
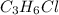 .
.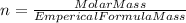
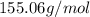

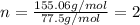
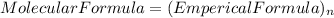 so, the molecular formula is:
so, the molecular formula is: