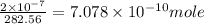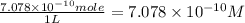Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1
You know the right answer?
The concentration of the alkane c20h42 (fm 282.56) in a particular sample of rainwater is 0.2 ppb. a...
Questions

Mathematics, 17.10.2019 15:30

Mathematics, 17.10.2019 15:30

Social Studies, 17.10.2019 15:30

History, 17.10.2019 15:30









Biology, 17.10.2019 15:30


Biology, 17.10.2019 15:30



History, 17.10.2019 15:30

English, 17.10.2019 15:30

Biology, 17.10.2019 15:30

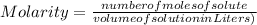 -(1)
-(1)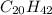 is determined as:
is determined as: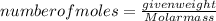 -(2)
-(2)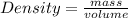
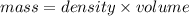 - (3)
- (3)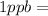
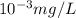
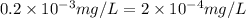
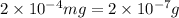 (As 1 mg =
(As 1 mg = 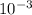 g).
g).