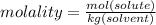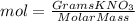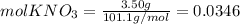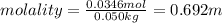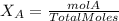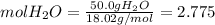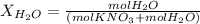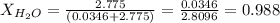Chemistry, 12.07.2019 12:00 Pizzapegasus1
Calculate the molality and mole fraction of water, respectively, of a solution that is made by dissolving 3.50 g of potassium nitrate in 50.0 g of water. the final volume of the solution is 56.0 ml.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
Calculate the molality and mole fraction of water, respectively, of a solution that is made by disso...
Questions

English, 10.12.2020 01:40



Mathematics, 10.12.2020 01:40

Mathematics, 10.12.2020 01:40

Chemistry, 10.12.2020 01:40



Medicine, 10.12.2020 01:40



Mathematics, 10.12.2020 01:40


English, 10.12.2020 01:40

Spanish, 10.12.2020 01:40

History, 10.12.2020 01:40


Arts, 10.12.2020 01:40