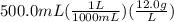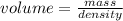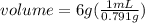Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
How many milliliters of pure liquid methanol (ch3oh, mw = 32.04 g/mol) are needed to prepare 500.0 m...
Questions

Mathematics, 17.12.2020 18:00

Computers and Technology, 17.12.2020 18:00







History, 17.12.2020 18:00





English, 17.12.2020 18:00

English, 17.12.2020 18:00

Mathematics, 17.12.2020 18:00


History, 17.12.2020 18:00

Biology, 17.12.2020 18:00

Mathematics, 17.12.2020 18:00

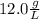 . This has to be made from a given pure liquid methanol with density
. This has to be made from a given pure liquid methanol with density 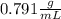 . It asks to calculate the volume of pure liquid methanol.
. It asks to calculate the volume of pure liquid methanol.