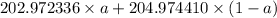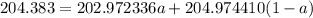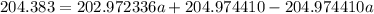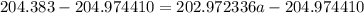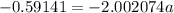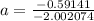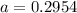Chemistry, 12.07.2019 12:00 tsedeneyaalemu2924
The atomic masses of 203tl and 205tl are 202.972336 and 204.974410 amu, respectively. the average atomic mass of thallium is 204.383 amu. calculate the natural abundances of these two isotopes.

Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
The atomic masses of 203tl and 205tl are 202.972336 and 204.974410 amu, respectively. the average at...
Questions

Advanced Placement (AP), 20.12.2020 22:30


Advanced Placement (AP), 20.12.2020 22:30









History, 20.12.2020 22:30

Biology, 20.12.2020 22:30

English, 20.12.2020 22:30

Mathematics, 20.12.2020 22:30



Social Studies, 20.12.2020 22:30

Mathematics, 20.12.2020 22:30

Advanced Placement (AP), 20.12.2020 22:30