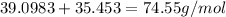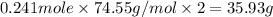Chemistry, 12.07.2019 09:00 mcdonaldmacy01
The compound cisplatin, pt(nh3)2cl2 , has been studied extensively as an antitumor agent. a. calculate the elemental percent composition by mass of cisplatin. b. cisplatin is synthesized as follows: k2ptcl4 (aq) + 2nh3(aq) â pt(nh3)2cl2 (s) + 2kcl (aq) what mass of cisplatin can be made from 100.g of k2ptcl4 and sufficient nh3? what mass of kcl is also produced?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2
You know the right answer?
The compound cisplatin, pt(nh3)2cl2 , has been studied extensively as an antitumor agent. a. calcula...
Questions


English, 29.01.2020 16:49


Biology, 29.01.2020 16:49



Mathematics, 29.01.2020 16:49



Biology, 29.01.2020 16:49

Mathematics, 29.01.2020 16:49



Computers and Technology, 29.01.2020 16:49


Biology, 29.01.2020 16:49


Chemistry, 29.01.2020 16:49

Biology, 29.01.2020 16:49

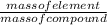 (1)
(1)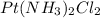 .
.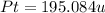
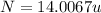
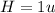
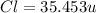
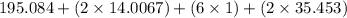 =
= 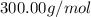
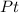 %
%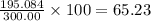 %.
%.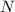 %
%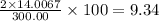 %
%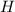 %
%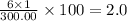 %
% %
%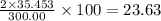 %
%
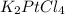 gives 1 mole of
gives 1 mole of 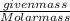
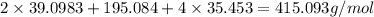
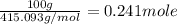 .
.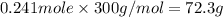
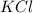 :
: