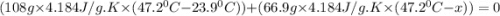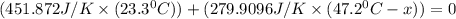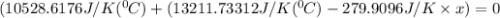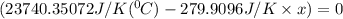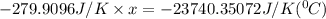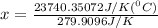Chemistry, 12.07.2019 09:00 Elephants12
When 108 g of water at a temperature of 23.9 °c is mixed with 66.9 g of water at an unknown temperature, the final temperature of the resulting mixture is 47.2 °c. what was the initial temperature of the second sample of water? (the specific heat capacity of liquid water is 4.184 j/g ⋅

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:10
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
When 108 g of water at a temperature of 23.9 °c is mixed with 66.9 g of water at an unknown temperat...
Questions



History, 29.04.2021 22:50







English, 29.04.2021 22:50





Mathematics, 29.04.2021 22:50


Mathematics, 29.04.2021 22:50



Mathematics, 29.04.2021 22:50

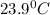
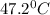
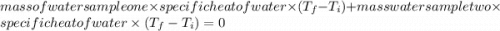
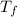 = final temperature
= final temperature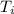 = initial temperature
= initial temperature