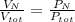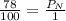Air is a mixture of gases that is about 78.0% n2 by volume. when air is at standard pressure and 25.0 ∘c, the n2 component will dissolve in water with a solubility of 4.88×10−4 m. what is the value of henry's law constant for n2 under these conditions? express your answer with the appropriate units. enter the unit m using the compound form mol/l.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
Air is a mixture of gases that is about 78.0% n2 by volume. when air is at standard pressure and 25....
Questions


Mathematics, 28.06.2019 06:00



Mathematics, 28.06.2019 06:00


Social Studies, 28.06.2019 06:00




Health, 28.06.2019 06:00


Mathematics, 28.06.2019 06:00


Mathematics, 28.06.2019 06:00


Biology, 28.06.2019 06:00