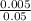Chemistry, 12.07.2019 02:30 jermainedwards
You wish to add 5 mg/l naocl as cl2 to a solution in a disinfection test, and you have a stock solution (household bleach) that contains 5% naocl by weight. assuming that the density of the stock solution is 1.0 g/ml, how many milliliters of bleach should you add to each liter of test solution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1


Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
You wish to add 5 mg/l naocl as cl2 to a solution in a disinfection test, and you have a stock solut...
Questions

Mathematics, 14.12.2020 23:00


Mathematics, 14.12.2020 23:00




English, 14.12.2020 23:00


Spanish, 14.12.2020 23:00

English, 14.12.2020 23:00


Mathematics, 14.12.2020 23:00


History, 14.12.2020 23:00

Mathematics, 14.12.2020 23:00

Mathematics, 14.12.2020 23:00


Mathematics, 14.12.2020 23:00

English, 14.12.2020 23:00

Mathematics, 14.12.2020 23:00

 x B g
x B g