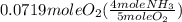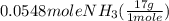Chemistry, 12.07.2019 02:30 Thejollyhellhound20
One of the steps in the commercial process for converting ammonia to nitric acid is the conversion of nh3 to no. 4 nh3(g) + 5 o2(g) → 4 no(g) + 6 h2o(g) in a certain experiment, 1.90 g of nh3 reacts with 2.30 g of o2. (a) which is the limiting reactant? o2 nh3 (b) how many grams of no and of h2o form? 1.73 g no 1.55 g h2o (c) how many grams of the excess reactant remain after the limiting reactant is completely consumed? g (d) show that your calculations in parts (b) and (c) are consistent with the law of conservation of mass. mass of products + excess reactant g total mass of reactants g

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Zinc + lead(ii) nitrate yield zinc nitrate + leadwhat's the chemical equation for this?
Answers: 1

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
You know the right answer?
One of the steps in the commercial process for converting ammonia to nitric acid is the conversion o...
Questions

Mathematics, 20.12.2020 01:00


History, 20.12.2020 01:00






History, 20.12.2020 01:00

Social Studies, 20.12.2020 01:00


Advanced Placement (AP), 20.12.2020 01:00

Physics, 20.12.2020 01:00


Mathematics, 20.12.2020 01:00


Mathematics, 20.12.2020 01:00


Mathematics, 20.12.2020 01:00

Physics, 20.12.2020 01:00

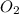 , (b) 1.73 g NO and 1.55 g
, (b) 1.73 g NO and 1.55 g 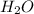 , (c) 0.932 g of
, (c) 0.932 g of 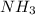 , (d) yes, the results are consistent with law of conservation of mass.
, (d) yes, the results are consistent with law of conservation of mass.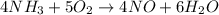
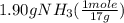 = 0.112 mole
= 0.112 mole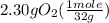 = 0.0719 mole
= 0.0719 mole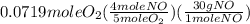 = 1.73 g of NO
= 1.73 g of NO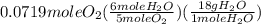 = 1.55 g of
= 1.55 g of