
Chemistry, 12.07.2019 02:30 Snowball080717
At a certain temperature, 0.740 mol of so3 is placed in a 4.00 l container. so_{3}(g)\rightleftharpoons 2so_{2}(g)+o_{2}(g) at equilibruim, 0.190 mol of o2 is present. calculate kc.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3


Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
At a certain temperature, 0.740 mol of so3 is placed in a 4.00 l container. so_{3}(g)\rightleftharpo...
Questions









Computers and Technology, 24.01.2020 22:31











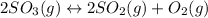
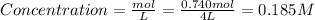

![k_{c} = \frac{[SO_{2}]^{2} [O_{2}]}{[SO_{3}]^{2}}](/tpl/images/0079/3196/d91b8.png)







