
Chemistry, 12.07.2019 01:30 nefertiri64
In a reaction where a + b ab, what does an equilibrium position of a= 14%, b = 14%, and ab= 72% indicate about the reaction? a. because a and b are equal, the reaction has reached equilibrium. b. there is more a and b than ab at equilibrium, and the reaction favors the reactants. c. there is more ab than a and b at equilibrium, and the reaction favors the products. d. there is more ab than a and b at equilibrium, and the reaction favors the reactants. e. the reaction is not at equilibrium.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 09:00
Individuals within populations exhibit some diversity. as a result of possessing slightly different traits, some individuals are better able to survive and reproduce than others. if these individuals changes in the characteristics of the population may occur over time. the cumulative change in these characteristics is known as
Answers: 3
You know the right answer?
In a reaction where a + b ab, what does an equilibrium position of a= 14%, b = 14%, and ab= 72% indi...
Questions

Health, 03.11.2020 02:10

History, 03.11.2020 02:10


Mathematics, 03.11.2020 02:10

Mathematics, 03.11.2020 02:10



World Languages, 03.11.2020 02:10




Mathematics, 03.11.2020 02:10




Biology, 03.11.2020 02:10

History, 03.11.2020 02:10

Biology, 03.11.2020 02:10


 is defined as the equilibrium constant. It is defined as the ratio of concentration of products to the concentration of reactants.
is defined as the equilibrium constant. It is defined as the ratio of concentration of products to the concentration of reactants.

![K_c=\frac{[AB]}{[A][B]}](/tpl/images/0079/1606/5faa1.png)
![K_c=\frac{[0.72]}{[0.14][0.14]}=36.7](/tpl/images/0079/1606/2d664.png)
 ; then the reaction is product favored.
; then the reaction is product favored.
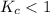 ; then the reaction is reactant favored.
; then the reaction is reactant favored.
 ; then the reaction is in equilibrium.
; then the reaction is in equilibrium.


