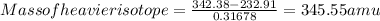Chemistry, 12.07.2019 00:00 aylengarcia090
Anew element was recently discovered and found to have an atomic weight of 342.38 amu. this element has two isotopes, the lighter of which has a mass of 340.91 amu and an abundance of 68.322%. what is the mass of the heavier isotope?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Microtubular fibers that assist in the movement of chromosomes during nuclear division in conjunction with proteins, makes up the small and large organelle pieces that assemble prior to translation three-base nucleotide sequence that can complementarily pair with the functional transcript, allowing a particular material to be brought to a ribosome particular time in the cell cycle when the cell’s systems determine if the cellular conditions are appropriate to continue through the cycle time in the cell’s cycle when proteins are made and organelles are duplicated enzyme that allows proper nucleotide bonding during transcription specific dna sequence which will initiate gene transcription division of the cell’s cytoplasm specific bond that forms between two amino acids when a carboxyl group binds to a amino group three-base sequence that does not code for a particular amino acid a paired organelle which facilitates the formation of movement microtubules time in the cell’s cycle when the microtubular structures exert an equal pressure on the cell’s genetic material
Answers: 2

Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3

Chemistry, 23.06.2019 06:30
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1

Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1
You know the right answer?
Anew element was recently discovered and found to have an atomic weight of 342.38 amu. this element...
Questions


Mathematics, 19.01.2021 23:10






Mathematics, 19.01.2021 23:10

Health, 19.01.2021 23:10

History, 19.01.2021 23:10

Mathematics, 19.01.2021 23:10


Mathematics, 19.01.2021 23:10


Computers and Technology, 19.01.2021 23:10

Mathematics, 19.01.2021 23:10

Biology, 19.01.2021 23:10

Mathematics, 19.01.2021 23:10

Biology, 19.01.2021 23:10