
(a) a 0.2 m potassium hydroxide solution is titrated with a 0.1 m nitric acid solution. (i) balanced equation: (ii)what would be observed if the solution was titrated well past the equivalence point using bromthymolblue as the indicator? (bromthymol blue is yellow in acidic solution and blue in basic solution.)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1
You know the right answer?
(a) a 0.2 m potassium hydroxide solution is titrated with a 0.1 m nitric acid solution. (i) balanced...
Questions

Mathematics, 24.02.2021 21:20

Mathematics, 24.02.2021 21:20





History, 24.02.2021 21:20




Mathematics, 24.02.2021 21:20





Mathematics, 24.02.2021 21:20

Biology, 24.02.2021 21:20


Chemistry, 24.02.2021 21:20

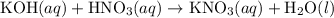
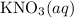 (and water) at the equivalence point. Both potassium hydroxide and nitric acid exist as strong electrolytes. As a result,
(and water) at the equivalence point. Both potassium hydroxide and nitric acid exist as strong electrolytes. As a result, 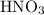 is added to the initially-basic solution. PH value of the solution would keep decreasing as the volume of the acid added increases. The final solution would be acidic as it contains not only water and
is added to the initially-basic solution. PH value of the solution would keep decreasing as the volume of the acid added increases. The final solution would be acidic as it contains not only water and 

