
Chemistry, 12.07.2019 00:00 josephfoxworth
Compound x is twice as soluble in diethyl ether as it is ethanol and is only sparingly soluble in water. compound y is soluble in water and etanol, but only sparingly soluble diethyl ether. you are given a solid mixture of x and y. briefly describe how you would separate x and y using liquid-liquid extraction?

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1

Chemistry, 24.06.2019 01:00
Watch the video to learn about the process of hydrogen bonding. hydrogen bonding is a type of intermolecular force that exists between polar covalent molecules, one of which has a hydrogen atom that is bonded to a small and extremely electronegative element, specifically an n, o, or f atom, on the other molecule. hydrogen bonding is a subset of dipole-dipole forces.
Answers: 1

Chemistry, 24.06.2019 01:30
Give four examples of how either some or all of the organisms in this activity are related to one another based on how they are classified.
Answers: 2
You know the right answer?
Compound x is twice as soluble in diethyl ether as it is ethanol and is only sparingly soluble in wa...
Questions



History, 05.01.2021 16:50




Mathematics, 05.01.2021 16:50






Social Studies, 05.01.2021 16:50


Mathematics, 05.01.2021 16:50



English, 05.01.2021 16:50

Health, 05.01.2021 16:50

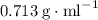 as opposed to
as opposed to 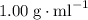 ,) it would form the upper layer of its mixture with water. Separate the mixture with a separatory funnel. The question stated that both X and Y are under the solid state while the two solvents are both liquidsm implying that the boiling points of both species are higher than that of their respective solvent. Therefore heat the solutions till all solvent had evaporated to obtain X and Y.
,) it would form the upper layer of its mixture with water. Separate the mixture with a separatory funnel. The question stated that both X and Y are under the solid state while the two solvents are both liquidsm implying that the boiling points of both species are higher than that of their respective solvent. Therefore heat the solutions till all solvent had evaporated to obtain X and Y. 

