

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
You know the right answer?
Dimethyl sulfoxide [(ch3)2so], also called dmso, is an important solvent that penetrates the skin, e...
Questions


Social Studies, 27.09.2021 06:00

Arts, 27.09.2021 06:10

Mathematics, 27.09.2021 06:10



History, 27.09.2021 06:10


Social Studies, 27.09.2021 06:10



Mathematics, 27.09.2021 06:10

Mathematics, 27.09.2021 06:10



Mathematics, 27.09.2021 06:10

History, 27.09.2021 06:10


History, 27.09.2021 06:10

Computers and Technology, 27.09.2021 06:10

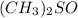 . Molar mass of dimethyl sulfoxide is 78.13 g/mol. Calculate number of moles as follows:
. Molar mass of dimethyl sulfoxide is 78.13 g/mol. Calculate number of moles as follows: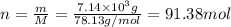
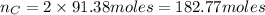
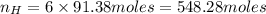
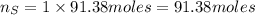
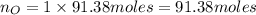
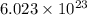 atoms thus, number of atoms can be calculated as:
atoms thus, number of atoms can be calculated as: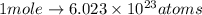
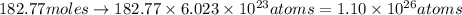
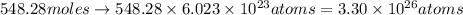
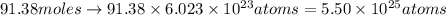
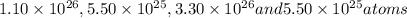 respectively.
respectively.

