
Chemistry, 11.07.2019 23:30 steffweikeloyrt00
Find the final equilibrium temperature when 15.0 g of milk at 13.0 degrees c is added to 148 g of coffee with a temperature of 88.3 degrees c. assume the specific heats of coffee and milk are the same as for water (c = 4.19 j/g•c), and disregard the heat capacity of the container.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:40
What type of solution is formed if 10 g of kclo3 are dissolved in 100g of water at 30
Answers: 2

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
Find the final equilibrium temperature when 15.0 g of milk at 13.0 degrees c is added to 148 g of co...
Questions



Mathematics, 02.07.2019 11:00


Mathematics, 02.07.2019 11:00

Mathematics, 02.07.2019 11:00

Biology, 02.07.2019 11:00

Social Studies, 02.07.2019 11:00

Mathematics, 02.07.2019 11:00

History, 02.07.2019 11:00

Geography, 02.07.2019 11:00


Spanish, 02.07.2019 11:00

Mathematics, 02.07.2019 11:00



Mathematics, 02.07.2019 11:00

Mathematics, 02.07.2019 11:00

Mathematics, 02.07.2019 11:00

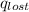 =
=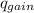 ,
, 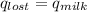 +
+ 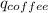
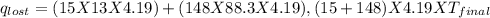 =(15X13X4.19)+(148X88.3X4.19)
=(15X13X4.19)+(148X88.3X4.19)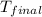 = 81.37 ° C
= 81.37 ° C

