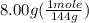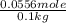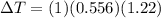Chemistry, 11.07.2019 23:00 kylucienne
The boiling point of pure ethanol, c2h5oh, is 78.4 ∘c. its boiling point elevation constant is 1.22 °c/m. what is the boiling point of a solution formed by dissolving 8.00 g of alpha-naphthol (c10h7oh) in 100.0 g ethanol. 91.3 °c 79.1°c 97.6 °c 78.5°c

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
The boiling point of pure ethanol, c2h5oh, is 78.4 ∘c. its boiling point elevation constant is 1.22...
Questions


English, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01






Biology, 16.10.2020 15:01



Biology, 16.10.2020 15:01

Social Studies, 16.10.2020 15:01

Computers and Technology, 16.10.2020 15:01


Social Studies, 16.10.2020 15:01

Chemistry, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01

Chemistry, 16.10.2020 15:01

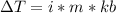
 is the elevation in boiling point
is the elevation in boiling point