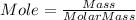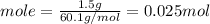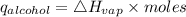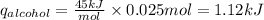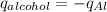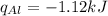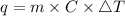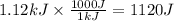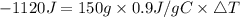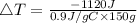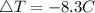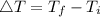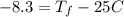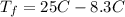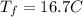Chemistry, 11.07.2019 22:30 reesewaggoner8
Consider 1.5g of rubbing alcohol (c3h8o) placed on a 150.g block of aluminum at 25 c. if all of the rubbing alcohol evaporates at 25 c, what is the final temperature of the aluminum?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
Consider 1.5g of rubbing alcohol (c3h8o) placed on a 150.g block of aluminum at 25 c. if all of the...
Questions



Mathematics, 28.03.2021 03:40


History, 28.03.2021 03:40


Mathematics, 28.03.2021 03:40

Mathematics, 28.03.2021 03:40


Chemistry, 28.03.2021 03:40

History, 28.03.2021 03:40

Mathematics, 28.03.2021 03:40

Mathematics, 28.03.2021 03:40


Computers and Technology, 28.03.2021 03:40


Mathematics, 28.03.2021 03:40


Mathematics, 28.03.2021 03:50