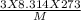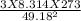Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3
You know the right answer?
At 273 k and 1.00 x 10^-2 atm, the density of a gas is 1.24 x 10^-5 g/cm3. a. find vrms for the gas...
Questions


History, 28.01.2020 10:31

Mathematics, 28.01.2020 10:31

Mathematics, 28.01.2020 10:31





English, 28.01.2020 10:31

Mathematics, 28.01.2020 10:31



Mathematics, 28.01.2020 10:31

Geography, 28.01.2020 10:31


Mathematics, 28.01.2020 10:31

Biology, 28.01.2020 10:31



 = √
= √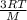 =√
=√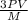 =√
=√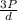 , where, R= Universal gas constant, T= Absolute temperature, P= Pressure, V= Volume of gas, d= Density of gas.
, where, R= Universal gas constant, T= Absolute temperature, P= Pressure, V= Volume of gas, d= Density of gas.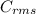 =√
=√