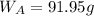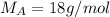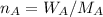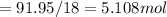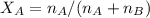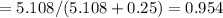A8.05 % ch3oh(aq) has a density of 0.976 g/ml at 18°c what is the mole fraction of the solvent in the solution? 0.953 8.05 91.95 0.0469
2) an aqueous solution of cesium chloride is prepared by dissolving 52.3 g cesium chloride in 60.0g of water at 25°c. the volume of this solution is 63.3 ml . what is the molality of the solution?
0.311 m
4.91 m
2.77 m
5.18 m
3) an aqueous solution of cesium chloride is prepared by dissolving 52.3 g cesium chloride in 60.0g of water at 25°c. the volume of this solution is 63.3 ml . what is the molarity of the solution?
4.91 m
2.69 m
5.18 m
2.77 m
4) the vapor pressure of ethanol, c2h5oh is 100.0 torr at 35 °c. calculate the vapor pressure of the solution formed by dissolving 28.8 g of alpha naphthol, c10h8o, in
36.8 g of c2h5oh. assume alpha naphthol to be nonvolatile at this temperature.
20.0 torr
43.9 torr
80.0 torr
56.1 torr
5) both ethanol, c2h5oh and propanol, c3h7oh, are volatile. at 35 °c, the vapor pressure of pure ethanol is 100 torr and that of propanol is 37.6 torr. what is the vapor pressure at this temperature of a solution is formed by mixing 36.9 g of ethanol and 12.0 g propanol.
15.3 torr
50.1 torr
84.7 torr
87.5 torr
the boiling point of pure ethanol, c2h5oh, is 78.4 latex: ^\circ ∘ c. its boiling point elevation constant is 1.22 °c/m. what is the boiling point of a solution formed by dissolving 8.00 g of alpha-naphthol (c10h7oh) in 100.0 g ethanol.
91.3 degrees centigrade
79.1 degrees centigrade
97.6 degrees centigrade
78.5 degrees centigrade
the freezing point of ccl4 is -22.92°c. calculate the freezing point of the solution prepared by dissolving 17.5g of pyrazine (c4h4n2) in 1250g of ccl4. the freezing point depression constant for ccl4 is 29.8 °c/m.
-22.50
-23.34
-17.71
-28.13
consider the following aqueous solutions:
a. 0.10 m nh4no3,
b. 0.10 m fe(no3)3
c. 0.10 m ba(no3)2
d. 0.10 m nh2conh2
arrange the following in increasing order (smallest to largest) order of osmotic pressure
c < b < a < d
a < d < c < b
d < a < c < b
a < c < b < d
consider the following aqueous solutions:
a. 0.10 m nh4no3,
b. 0.10 m fe(no3)3
c. 0.10 m ba(no3)2
d. 0.10 m nh2conh2
arrange the following in increasing order (smallest to largest) order of freezing point. the freezing point of pure water is 0.00 ∘ c and its freezing point depression constant is 1.86 ∘ c/m
d < a < c < b
b < c < a < d
c < b < a < d
a < c < b < d
consider the following aqueous solutions:
a. 0.10 m nh4no3,
b. 0.10 m fe(no3)3
c. 0.10 m ba(no3)2
d. 0.10 m nh2conh2
arrange the following in increasing order (smallest to largest) order of normal boiling point. the normal boiling point of pure water is 100.00 ∘ c and its boiling point elevation constant is 0.512 ∘ c/m
c < b < a < d
d < a < c < b
a < c < b < d
b < c < a < d
a solution is prepared by dissolving 1.22 g of compound in enough water to make up 262 ml in volume. the osmotic pressure of the solution is found to be 30.3 torr at
35.0 °c. calculate the molar mass of the compound.
257 g/mol
2950 g/mol
3.88 g/mol
44.7 g/mol
i tried to solve them all but i keep getting wrong answers can anyone

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1

Chemistry, 23.06.2019 10:30
Describe the hybridization of each carbon and nitrogen atom in each of the following structures
Answers: 1
You know the right answer?
A8.05 % ch3oh(aq) has a density of 0.976 g/ml at 18°c what is the mole fraction of the solvent in th...
Questions

Mathematics, 19.03.2021 06:20



Mathematics, 19.03.2021 06:20

English, 19.03.2021 06:20


Chemistry, 19.03.2021 06:20






Mathematics, 19.03.2021 06:20

Chemistry, 19.03.2021 06:20

English, 19.03.2021 06:20



Mathematics, 19.03.2021 06:20

Mathematics, 19.03.2021 06:20

Mathematics, 19.03.2021 06:20