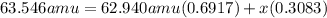Chemistry, 11.07.2019 19:30 oranjejuice
Copper has two naturally occurring isotopes and an atomic mass of 63.546 amu. cu-63 has a mass of 62.940 amu and an abundance of 69.17%. what is the identity and percent abundance of copper's other isotope? (type your answer for identity using the format cl-35 for chlorine-35.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1


Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1
You know the right answer?
Copper has two naturally occurring isotopes and an atomic mass of 63.546 amu. cu-63 has a mass of 62...
Questions

Chemistry, 10.12.2020 22:20

History, 10.12.2020 22:20



Mathematics, 10.12.2020 22:20

Mathematics, 10.12.2020 22:20

Biology, 10.12.2020 22:20

Biology, 10.12.2020 22:20


Mathematics, 10.12.2020 22:20


Mathematics, 10.12.2020 22:20


Biology, 10.12.2020 22:20

Biology, 10.12.2020 22:20

Computers and Technology, 10.12.2020 22:20


Biology, 10.12.2020 22:20

Mathematics, 10.12.2020 22:20

Business, 10.12.2020 22:20