Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
You know the right answer?
Consider the reaction, 2 d(g) + 3 e(g) + f(g) => 2 g(g) + h(g) when e is decreasing at 0.16 mol/...
Questions


English, 23.12.2021 14:00

Mathematics, 23.12.2021 14:00


History, 23.12.2021 14:00

English, 23.12.2021 14:00

SAT, 23.12.2021 14:00

Social Studies, 23.12.2021 14:00



English, 23.12.2021 14:00



Social Studies, 23.12.2021 14:00

Business, 23.12.2021 14:00



Chemistry, 23.12.2021 14:00

Mathematics, 23.12.2021 14:00

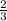 moles of g formed
moles of g formed