
Chemistry, 11.07.2019 19:00 hayesvolcano
A1.42-g sample of a pure compound with formula m2so4 was dissolved in water and treated with an ex- cess of aqueous calcium chloride, resulting in the precipi- tation of all the sulfate ions as calcium sulfate. the pre- cipitate was collected, dried, and found to weigh 1.36 g. determine the atomic mass of m and identify m.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2


Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
A1.42-g sample of a pure compound with formula m2so4 was dissolved in water and treated with an ex-...
Questions









Computers and Technology, 13.05.2021 20:40



Spanish, 13.05.2021 20:40

History, 13.05.2021 20:40






Computers and Technology, 13.05.2021 20:40

Mathematics, 13.05.2021 20:40

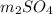 with calcium chloride can be shown as-
with calcium chloride can be shown as-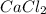 →
→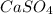 ↓+2mCl. The molecular weight of
↓+2mCl. The molecular weight of 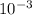 moles of calcium sulfate.
moles of calcium sulfate.

