Chemistry, 11.07.2019 19:00 bbgirl2505
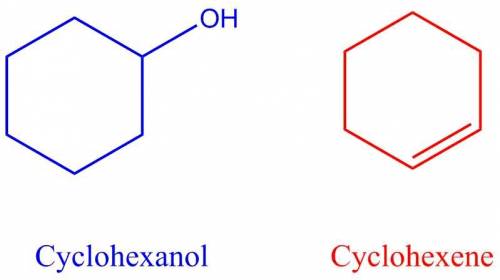
Areaction was performed in which 4.0 g of cyclohexanol was reacted with an acid catalyst to obtain 2.8 g of cyclohexene. calculate the percent yield for this reaction.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 23.06.2019 11:30
Place the following substances in order of ph from lowest ph to highest. a. neutral compounds, bases, acids b. acids, neutral compounds, bases c. bases, acids, neutral compounds d. bases, neutral compounds, acids
Answers: 1
You know the right answer?
Areaction was performed in which 4.0 g of cyclohexanol was reacted with an acid catalyst to obtain 2...
Questions


Mathematics, 14.02.2020 02:01

History, 14.02.2020 02:01







Mathematics, 14.02.2020 02:01

Mathematics, 14.02.2020 02:01


Mathematics, 14.02.2020 02:02



History, 14.02.2020 02:02


Mathematics, 14.02.2020 02:02