
Chemistry, 11.07.2019 19:00 cjdolce9790
The element lead (pb) consists of four naturally occurring isotopes with atomic masses 203.97302,205.97444,206.97587, n 207.97663 amu. the relative abundances of these four isotopes are 1.4,24.1,22.1, and 52.4 %, respectively. write the most common isotope of lead in two ways

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
The element lead (pb) consists of four naturally occurring isotopes with atomic masses 203.97302,205...
Questions

History, 28.09.2019 21:30


Mathematics, 28.09.2019 21:30



Mathematics, 28.09.2019 21:30

Mathematics, 28.09.2019 21:30


Social Studies, 28.09.2019 21:30

Mathematics, 28.09.2019 21:30

Chemistry, 28.09.2019 21:30


Mathematics, 28.09.2019 21:30


English, 28.09.2019 21:30


Social Studies, 28.09.2019 21:30


Social Studies, 28.09.2019 21:30

Spanish, 28.09.2019 21:30

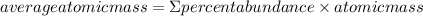
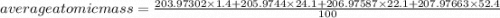
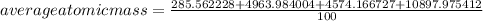
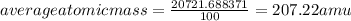
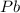 . The atomic number of
. The atomic number of 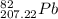 and Lead - 207.22 amu.
and Lead - 207.22 amu.

