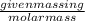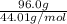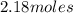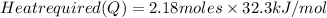Chemistry, 11.07.2019 19:00 kyrabrown33
Calculate the amount of heat required to completely sublime 96.0 g of solid dry ice (co2) at its sublimation temperature. the heat of sublimation for carbon dioxide is 32.3 kj/mol. calculate the amount of heat required to completely sublime 96.0 g of solid dry ice (co2) at its sublimation temperature. the heat of sublimation for carbon dioxide is 32.3 kj/mol.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1


Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
Calculate the amount of heat required to completely sublime 96.0 g of solid dry ice (co2) at its sub...
Questions

English, 07.07.2019 04:30



French, 07.07.2019 04:30

Chemistry, 07.07.2019 04:30

Biology, 07.07.2019 04:30



History, 07.07.2019 04:30


Chemistry, 07.07.2019 04:30

Mathematics, 07.07.2019 04:30

Chemistry, 07.07.2019 04:30



Chemistry, 07.07.2019 04:30


Social Studies, 07.07.2019 04:30

Physics, 07.07.2019 04:30

History, 07.07.2019 04:30

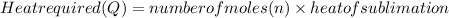 (1)
(1)