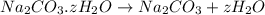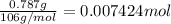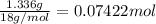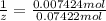Chemistry, 11.07.2019 19:00 ynclankaedon
Washing soda is a hydrated compound whose formula can be written na2co3 i zh2o, where z is the number of moles of h2o per mole of na2co3. when a 2.123 g sample of washing soda was heated at 130°c, all of the water of hydration was lost, leaving 0.787 g of anhydrous sodium carbonate. calculate the value of z.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:50
Achemical reaction (also known as a chemical change) produces substances that are chemically different from the starting materials. an example of a chemical reaction is the formation of water from hydrogen and oxygen gas.in a physical change, a substance changes its physical appearance but not its chemical identity. an example of physical change is the formation of liquid water from solid water, a familiar process called melting. physically, liquid water looks very different from solid water (ice) but the chemical identity, water, is the same for both. which of following changes that affect the composition of our atmosphere involve physical changes and which involve chemical reactions? oxygen gas changes to ozone during thunderstorms carbon dioxide is produced by the combustion of gasoline in an automobile engine. when coal, oil, and natural gas are decomposed in landsills they produce methane gas. freezing rain develops when a warm air mass overrides a cold air mass. fog forms from water vapor when the temperature drops below the dew point
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
Washing soda is a hydrated compound whose formula can be written na2co3 i zh2o, where z is the numbe...
Questions


Biology, 29.10.2020 07:30

History, 29.10.2020 07:30

Mathematics, 29.10.2020 07:30

Mathematics, 29.10.2020 07:30

Mathematics, 29.10.2020 07:30


English, 29.10.2020 07:30


Advanced Placement (AP), 29.10.2020 07:30

Mathematics, 29.10.2020 07:30

Mathematics, 29.10.2020 07:40


History, 29.10.2020 07:40





Arts, 29.10.2020 07:40

World Languages, 29.10.2020 07:40

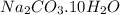 .
.