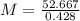The element antimony has an atomic weight of 122 and consists of two stable isotopes antimony-121 and antimony-123. the isotope antimony-121 has a mass of 121 amu and a percent natural abundance of 57.3 %. the isotope antimony-123 has a percent natural abundance of 42.8 %. what is the mass of antimony-123?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
The element antimony has an atomic weight of 122 and consists of two stable isotopes antimony-121 an...
Questions




Mathematics, 09.10.2019 13:30







Mathematics, 09.10.2019 13:30


Biology, 09.10.2019 13:30

Mathematics, 09.10.2019 13:30


Mathematics, 09.10.2019 13:30


Mathematics, 09.10.2019 13:30

Physics, 09.10.2019 13:30

Mathematics, 09.10.2019 13:30