
Choose the correct interpretation of this result in terms of the law of definite proportion. the composition of a substance depends on the total number of atoms and not on the composition of the mixture from which it was formed. the composition of a substance depends on the numbers of atoms of each element making up the compound (depends on the formula of the compound) and not on the composition of the mixture from which it was formed. the composition of a substance depends on the composition of the mixture from which it was formed and not on the numbers of atoms of each element making up the compound (not on the formula of the compound). the composition of a mixture depends on the numbers of atoms of each element making up the mixture and not on the composition of the substance from which it was formed.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3


Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2
You know the right answer?
Choose the correct interpretation of this result in terms of the law of definite proportion. the com...
Questions


Mathematics, 08.12.2020 20:30

Mathematics, 08.12.2020 20:30

Mathematics, 08.12.2020 20:30

Health, 08.12.2020 20:30




English, 08.12.2020 20:30

Mathematics, 08.12.2020 20:30

Chemistry, 08.12.2020 20:30

Mathematics, 08.12.2020 20:30

Computers and Technology, 08.12.2020 20:30


English, 08.12.2020 20:30

Biology, 08.12.2020 20:30




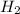 (g) +
(g) + 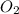 (g) = 2
(g) = 2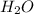 (l). Here the origin of the components i.e. hydrogen and oxygen does not matter it depends upon the number of atoms present in the reactant and product. Thus among all the other interpretations- The composition of a substance depends on the total number of atoms and not on the composition of the mixture from which it was formed. This is correct.
(l). Here the origin of the components i.e. hydrogen and oxygen does not matter it depends upon the number of atoms present in the reactant and product. Thus among all the other interpretations- The composition of a substance depends on the total number of atoms and not on the composition of the mixture from which it was formed. This is correct. 

