
Bromine reacts with nitric oxide to form nitrosyl bromide as shown in this reaction: br2(g) + 2 no(g) → 2 nobr(g) a possible mechanism for this overall reaction is shown below. no(g) + br2(g) br2(g) (fast step; keq = k1/k−1) k2 nobr(g) + no(g) → 2 nobr(g) (slow step) what is the rate law for formation of nobr in terms of reactants based on this mechanism

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1



Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2
You know the right answer?
Bromine reacts with nitric oxide to form nitrosyl bromide as shown in this reaction: br2(g) + 2 no(...
Questions



Mathematics, 18.11.2020 17:20


Mathematics, 18.11.2020 17:20

SAT, 18.11.2020 17:20

Mathematics, 18.11.2020 17:20

Computers and Technology, 18.11.2020 17:20

SAT, 18.11.2020 17:20





Mathematics, 18.11.2020 17:20



Mathematics, 18.11.2020 17:20



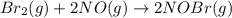
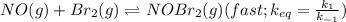
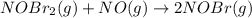 (slow step
(slow step 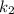 )
)![r_{1}=k_{1}[NO][Br_{2}]-k_{-1}[NOBr_{2}]](/tpl/images/0077/5023/9bc26.png)
![r_{2}=k_{2}[NOBr_{2}] [NO]](/tpl/images/0077/5023/6e659.png)
![[NOBr_{2}]](/tpl/images/0077/5023/48931.png) takes place in this reaction.
takes place in this reaction.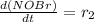
![k_{2}[NOBr_{2}][NO]](/tpl/images/0077/5023/433e8.png) (1)
(1)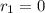
![k_{1}[NO][Br_{2}]= k_{-1}[NOBr_{2}]](/tpl/images/0077/5023/8345a.png)
![[NOBr_{2}] = \frac{k_{1}}{k_{-1}}[NO][Br_{2}]](/tpl/images/0077/5023/ee42f.png)
![\frac{d(NOBr)}{dt}=k_{2}[NOBr_{2}][NO]](/tpl/images/0077/5023/ff99b.png)
![k_{2} \frac{k_{1}}{k_{-1}}[NO][Br_{2}][NO]](/tpl/images/0077/5023/f5111.png)
![\frac{k_{1}k_{2}}{k_{-1}}[NO]^{2}[Br_{2}]](/tpl/images/0077/5023/427a8.png)
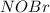 in terms of reactants is given by
in terms of reactants is given by 

