

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

You know the right answer?
Calculate the pressure exerted by ar for a molar volume of 0.470 l⋅mol−1 at 295 k using the van der...
Questions


Mathematics, 20.02.2020 22:47



Spanish, 20.02.2020 22:47











English, 20.02.2020 22:48


Biology, 20.02.2020 22:48


Mathematics, 20.02.2020 22:48

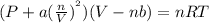 -(1)
-(1)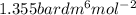 and b =
and b = 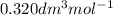
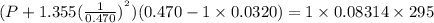
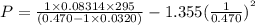
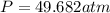
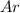 using the van der Waals equation is
using the van der Waals equation is 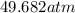 .
.


